In a groundbreaking experiment in the early 1950s, a scientist tried to re-create the conditions of early Earth in a test tube.
Stanley Miller added a few simple ingredients thought to be swirling in the young planet's atmosphere and oceans to interconnected flasks, applied heat and zapped them with electricity to simulate lightning. The findings quickly became famous: Out of this primordial soup emerged amino acids, the chemical building blocks of life.
The discovery kick-started a quest within chemistry and biology to devise experiments that could help answer one of the biggest scientific questions facing humanity: How did life on Earth begin?
Now, scientists at Ludwig Maximilian University of Munich have taken an exciting step forward by showing how more complex molecules crucial for life could have been synthesized from early Earth's basic ingredients.
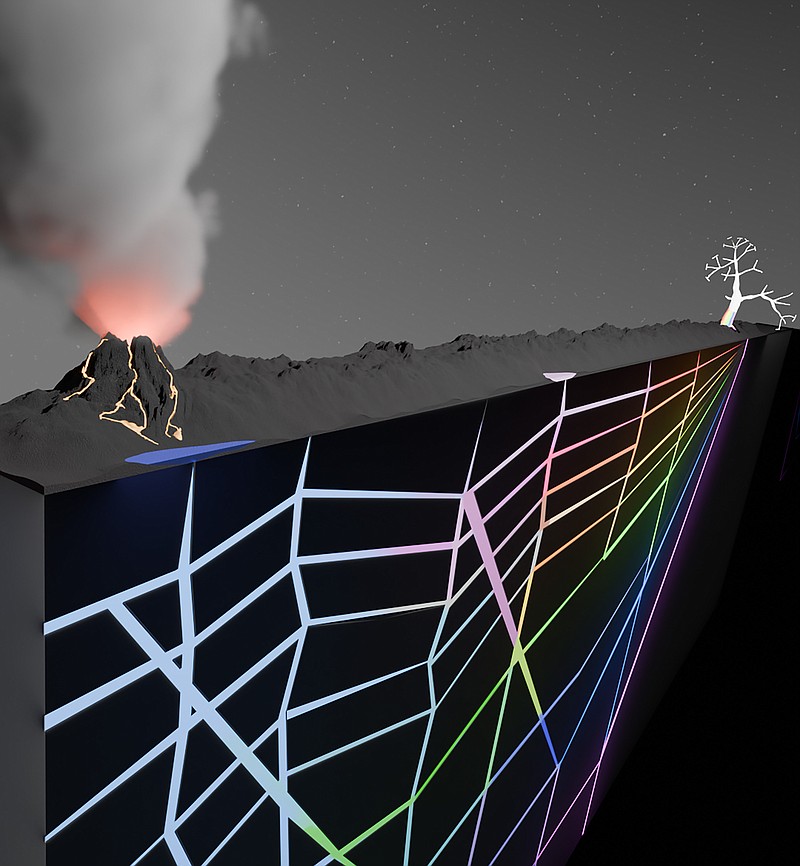
In their study, published in the journal Nature, the scientists swapped test tubes for tiny networks of branching cracks that resemble those that naturally form in rocks. They flowed water through the cracks, along with key chemical building blocks, then applied heat, mimicking a process akin to what might happen near hydrothermal vents in the ocean or in porous rocks near a geothermal pool.
They discovered that the heat flowing across these geologic networks sorted and filtered molecules, helping them create longer chains called biopolymers that are essential for life.
"It's a fantastic demonstration that simple physical processes can work to do this stuff," said Matthew Pasek, a geoscience professor at the University of South Florida who was not involved in the research.
Because the question of how life began is so big, it transcends the traditional boundaries that carve science into different disciplines. Chemists, biologists, astrophysicists and geologists all have a seat at the table when trying to answer the question.
Bridging those boundaries is what interested Christof Mast, a biophysicist at Ludwig Maximilian University of Munich, whose lab designed an experimental setup that would be somewhat closer to the conditions where the "prebiotic chemistry" that gave rise to life took place.
For decades, scientists have wrestled with the problem that early Earth wasn't a pristine laboratory, with beakers, impeccably timed purification steps and concentrated stocks of ingredients. It's one thing to re-create the chemistry of life in a lab, but experiments that are doable in a flask may be improbable at best under messy real-world conditions.
"You can think of the prebiotic Earth, this prebiotic soup, that is highly dilute, and all these different things react in a very uncontrolled way," Mast said.
One of the problems to date is that chemical reactions in the lab often result in side products that can start their own unwanted reactions, leaving scientists with only tiny amounts of the key material. So how did early Earth brew up enough of these building blocks for life to eventually blaze into existence?
To try to figure that out,the researchers cut branching networks of interconnected cracks into a tiny piece of aninert Teflon-like substance called FEP and sandwiched it between two sapphire plates. The sapphires were brought toprecisebutdifferent temperatures to create heat flux through the geologic network between them, mimicking the way that heat probably flowed on early Earth - perhaps near volcanoes or hydrothermal vents. Then, they flowed water and basic chemical building blocks through the crack network and observed what happened.
In one proof-of-concept experiment, they used glycine, the simplest amino acid, along with a substance called TMP that can react to link two glycine molecules.
Such reactions are difficult in water, Mast said, and TMP was very rare on early Earth. When they just mixed those ingredients together in a beaker, or in geologic cracks without heat, the amount of the more complex biopolymer they created was "vanishingly small," the researchers reported.
But when they applied a heat gradient to the cracks, it massively increased the production of the biopolymer.That's significant becausewhile amino acids are important, they are still far from life. Those same basic building blocks have been found on lifeless meteorites, for example.
"In order to get things the next level up, you have to start making the polymers - that's a fundamental step in making the next realm of life," Pasek said.
The setup can't weigh in on the ultimate question about how life began: Was it in a pond, as might have existed on Earth's surface, or near a hydrothermal vent akin to the ones found deep in the ocean? Heat flux across rock could occur in a multitude of geological settings, Mast said, and were probably "ubiquitous" on early Earth.
But the experimental setup can be used to test other questions about early chemistry on the planet. Mast is hoping to next create a network of cracks out of geologic materials, and to build larger networks of connected chambers.
The study is yet another reminder that elegant chemistry experiments can ignore a fundamental part of the primordial soup: the pot.
In 2021, a team of scientists found that in the famous 1950s experiment, the test tube itself - or rather, the borosilicate glass that it was made of - played a role in the results.
When those scientists repeated the experiment in a glass flask, a Teflon flask, and then in a Teflon flask with a bit of borosilicate glass, they found that the glass was a critical ingredient in catalyzing the reactions.
"In other words, for cooking the 'primordial soup,' the casserole is important," Juan Manuel García-Ruiz, a research professor at the Donostia International Physics Center in Spain who was involved in the experiment, wrote in an email. He praised the new work for its imaginative approachand, perhaps most importantly, for being "geologically plausible."
"It may not be the only mechanism, but it works and is ingenious, and above all, it is an experimental demonstration," García-Ruiz said. "I think that we need more experimental approaches to explore the geochemical context of the planet when life was born."